IIT JAM Question Paper with Solutions 2025: Chemistry
IIT JAM Chemistry memory based questions 2025 with solutions are mentioned below.
Q1. Acidity order of aqua complexes having coordination number 6 of $\mathrm{Al^{3+}}$, $\mathrm{Fe^{2+}}$, and $\mathrm{Fe^{3+}}$
Answer:
The acidity of aqua complexes depends on the charge density of the central metal ion. A higher charge and smaller ionic radius increase the polarisation of coordinated water molecules, enhancing acidity. Since $\mathrm{Fe^{3+}}$ has the highest charge density, it forms the most acidic complex.
$$
\mathrm{Fe^{3+} > Al^{3+} > Fe^{2+}}
$$
Q2. $\mathrm{^{12}C^{16}O}$ has a vibrational frequency of $3.8$. If oxygen is replaced by $\mathrm{^{18}O}$, what is the new frequency?
Answer:
The vibrational frequency is given by
$$
\nu = \frac{1}{2\pi}\sqrt{\frac{k}{\mu}}
$$
Thus,
$$
\frac{\nu_2}{\nu_1} = \sqrt{\frac{\mu_1}{\mu_2}}
$$
Reduced mass:
For $\mathrm{^{12}C^{16}O}$,
$$
\mu_1 = \frac{12 \times 16}{12 + 16} = \frac{192}{28} = 6.85
$$
For $\mathrm{^{12}C^{18}O}$,
$$
\mu_2 = \frac{12 \times 18}{12 + 18} = \frac{216}{30} = 7.2
$$
Now,
$$
\nu_2 = 3.8 \sqrt{\frac{6.85}{7.2}} = 3.71
$$
Q3. A $1.84\,\mathrm{g}$ mixture of $\mathrm{CaCO_3}$ is heated until constant weight. The residue weighs $0.96\,\mathrm{g}$. Find the percentage of $\mathrm{CaCO_3}$ in the mixture.}
Answer:
$$
\mathrm{CaCO_3 \rightarrow CaO + CO_2}
$$
$$
\frac{\text{Molar mass of CaO}}{\text{Molar mass of CaCO}_3} = \frac{56}{100} = 0.56
$$
$$
\text{Mass of CaCO}_3 = \frac{0.96}{0.56} = 1.71\,\mathrm{g}
$$
$$
\%\ \mathrm{CaCO_3} = \frac{1.71}{1.84} \times 100 = 92.93\%
$$
Q4. If $\mathrm{P_4O_{10}}$ is hydrolysed and then heated above $300^\circ\mathrm{C}$, what is the final product?
Answer:
$$
\mathrm{P_4O_{10} + 6H_2O \rightarrow 4H_3PO_4}
$$
$$
\mathrm{2H_3PO_4 \rightarrow H_4P_2O_7 + H_2O}
$$
$$
\mathrm{H_4P_2O_7 \rightarrow 2HPO_3 + H_2O}
$$
Final product: $\mathrm{HPO_3}$ (metaphosphoric acid).
Q5. Write the acidity order for $[\mathrm{Fe(NH_3)_6}]^{2+}$, $[\mathrm{Fe(NH_3)_6}]^{3+}$, and $[\mathrm{Al(NH_3)_6}]^{3+}$.
Answer:
Acidity depends on charge density and ionic radius of the central metal ion. Greater polarisation of $\mathrm{NH_3}$ increases acidity.
$$
\mathrm{[Al(NH_3)_6]^{3+} > [Fe(NH_3)_6]^{3+} > [Fe(NH_3)_6]^{2+}}
$$
Q6. An electron is accelerated through a $10\,\mathrm{kV}$ potential. Find its de Broglie wavelength.
Answer:
$$
\lambda = \frac{h}{\sqrt{2meV}}
$$
$$
2meV = 2 \times 9.1 \times 10^{-31} \times 1.602 \times 10^{-19}
= 2.92 \times 10^{-23}
$$
$$
\sqrt{2meV} = 5.41 \times 10^{-12}
$$
$$
\lambda = \frac{6.626 \times 10^{-34}}{5.41 \times 10^{-12}}
= 1.25 \times 10^{-10}\,\mathrm{m}
= 0.125\,\mathrm{nm}
$$
Q7. Number of stereoisomers
Answer:
Insufficient information. The compound or structure must be specified.
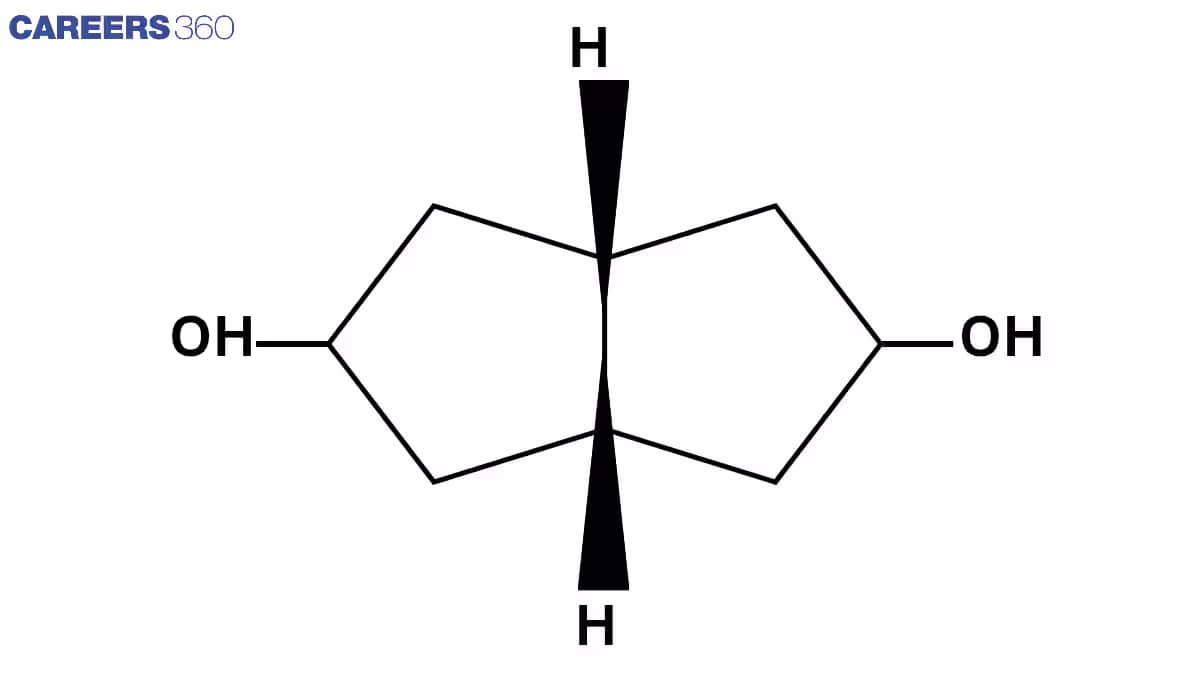
Ans. There are 3 optical centre
No. of Sterioisomer is 2n=23=8
IIT JAM 2026 Preparation Tips – How to Score High
This section provides essential IIT JAM 2026 preparation tips, helping aspirants plan effectively, strengthen conceptual understanding, and improve speed and accuracy to score high in the exam.
Understand the Exam Pattern and Syllabus
Start by thoroughly understanding the IIT JAM 2026 exam pattern, marking scheme, and subject-wise syllabus. Each subject includes MCQs, Multiple Select Questions (MSQs), and Numerical Answer Type (NAT) questions. Knowing the pattern helps in effective planning and focusing on high-weightage topics.
Create a Subject-Wise Study Plan
Divide your preparation across all subjects and chapters. Allocate more time to difficult or high-scoring topics while maintaining a balance between theory and numerical problem practice. A structured timetable ensures no topic is left untouched and reduces last-minute stress.
Strengthen Conceptual Clarity
Focus on understanding fundamental principles, derivations, and formulas rather than rote memorization. Use NCERT textbooks and standard reference books to build a strong foundation in Physics, Mathematics, Chemistry, Biotechnology, or Economics.
Practice Previous Years’ Question Papers
Solving past IIT JAM papers helps identify question trends, difficulty levels, and frequently tested topics. It improves problem-solving speed, builds confidence, and helps manage time effectively during the exam.
Take Regular Mock Tests
Full-length mock tests simulate the real IIT JAM environment. They enhance speed, accuracy, and exam confidence. Analyze mistakes after each test and revise weak areas to maximize scoring potential.
Focus on Numerical Answer Type (NAT) Questions
NAT questions carry significant marks and require precise calculation. Practice step-by-step numerical problems daily, memorize key formulas, and use shortcuts for faster and accurate solving.
Revise Important Topics Regularly
Revision is essential. Maintain concise notes, formula sheets, and topic summaries. Focus on high-yield chapters that are frequently tested and revise them multiple times before the exam.
Manage Time Effectively During Preparation
Time management is crucial during preparation and the exam. Solve questions within a time limit, prioritize easier questions first, and allocate remaining time to difficult problems. Avoid spending too long on a single question.


