UPES M.Sc Admissions 2026
Last Date to Apply: 25th Feb | Ranked #45 Among Universities in India by NIRF | 1950+ Students Placed 91% Placement, 800+ Recruiters
IIT JAM Chemistry Question Paper 2025: The Indian Institute of Technology Joint Admission Test for Masters or IIT JAM is one of India's most popular postgraduate examinations. It is estimated that more than 50,000 students appear for the IIT JAM 2025 examination. Various prestigious institutions in the country such as the IITs, IIScs, NITs, DIAT and IIEST accept a valid IIT JAM score.
This Story also Contains
The level of difficulty of the IIT JAM Chemistry examination was between moderate to difficult levels as compared to the previous year’s IIT JAM Chemistry Question Papers. Aspiring candidates should focus on previous years’ papers to get a better grasp of the IIT JAM Chemistry question paper 2025 pattern. The IIT JAM Chemistry question paper 2025 included questions from various topics like organic chemistry, physical chemistry, and inorganic chemistry, in line with the updated syllabus.
IIT JAM Chemistry memory-based questions with solutions are mentioned below.
Q 1. Acidity order of aqua complexes having CN₆ of Al³⁺, Fe²⁺, and Fe³⁺?
Ans, The acidity of aqua Complexes depends on the charge density of $u$ Central metal ion. $\mathrm{Fe}^{3+}$ has highest charge density we to smallest ionic radius, making Complex most acidic.
$\mathrm{Fe}^{3 t}>\mathrm{Al}$ bt $>\mathrm{Fe}^{2 t}$ - acidic order.
Q 2. C₁₂O₁6 having frequency 3.8 has O₁₈. What is the new frequency?
Ans. $
\begin{aligned}
& v=\frac{1}{2 \pi} \sqrt{\frac{k}{\mu}} \text { or } \frac{v_2}{v_1}=\sqrt{\frac{\mu_1}{\mu_2}} \\
& \mu=\frac{m_1 m_2}{m_1+m_2}
\end{aligned}
$
For $c^{12}-0^{16}$
$
\mu=\frac{12 \times 16}{12+16}=\frac{192}{28}=6.85
$
For $C^{12}-0^{18} \quad A=\frac{12 \times 18}{12+18}=\frac{216}{30}=7.2$
$
\begin{aligned}
& \frac{v_2}{v_1}=\sqrt{\frac{6.0 .57}{7.2}} \\
& v_2=3.71
\end{aligned}
$
Q 3. A 1.84 g mixture of CaCO₃ reacts until weight loss. The residual weight is 0.96 g. What is the percentage composition of CaCO₃ in the mixture?
Ans. $\mathrm{CaCO}_3 \longrightarrow \mathrm{CaO}+\mathrm{CO}_2$
$\begin{aligned} n & =\frac{\text { Molar mass of } \mathrm{CaO}}{\text { Molar mass of } \mathrm{CaCO}_3} \\ & =\frac{56}{100}=0.56\end{aligned}$
$\begin{aligned} & \text { Amount of } \mathrm{CaCO}_3 \\ & \begin{aligned} \frac{\text { mass of }{ }^2 \mathrm{CO}}{n} & =\text { Amount } \\ \frac{0.96}{0.56} & =1.71 \mathrm{~g} \\ \% \text { of } \mathrm{CaCO}_3 & =\left(\frac{1.71}{1.84}\right) \times 100 \\ & =92.93 \%\end{aligned}\end{aligned}$
Q 4. If P₄O₁₀ is hydrolyzed and then heated above 300°C. What will be the product?
Ans. $\begin{aligned} & \text { Above } 300^{\circ} \mathrm{C} \text { give }\left(\mathrm{HPO}_3\right) \\ & \mathrm{P}_4 \mathrm{O}_1 \mathrm{O}+6 \mathrm{H}_2 \mathrm{O} \rightarrow 4 \mathrm{H}_3 \mathrm{PO}_4 \\ & 2 \mathrm{H}_3 \mathrm{PO}_4 \rightarrow \mathrm{H}_4 \mathrm{P}_2 \mathrm{O}_7+\mathrm{H}_2 \mathrm{O} \\ & \mathrm{H}_4 \mathrm{P}_2 \mathrm{O} \rightarrow 2 \mathrm{HPO}_3+\mathrm{H}_2 \mathrm{O}\end{aligned}$
Q 5. Write the acidity order for [fe(NH3)6]+2,[fe(NH3)6]+3,[Al (NH3)6]+3
Ans. The acidity of Coordination Complexes depends on the charge density of Central metal ion. A higher charge density and 8 maller ionic radius increase the polarisation of the ligand $\left(\mathrm{NH}_3\right)$.
$
\mathrm{Al}^{3+}>\mathrm{Fe}^{3+}>\mathrm{Fe}^{2 t}-\text { acidic order }
$
Q 6. Electron is Accelerated through 10 KV Potential, Then De-broglie wave length (lamba) =?
Ans. $\begin{aligned}
\lambda & =\frac{h}{\sqrt{2 m e V}} \\
2 m_e V & =2 \times\left(9.1 \times 10^{-31}\right) \times\left(1.602 \times 10^{-19}\right) \\
& =2.92 \times 10^{-2} 3 \mathrm{~J} / \mathrm{kg} \\
\text { Ine } \sqrt{2 m_e V} & =\sqrt{2.92 \times 10^{-23}} \\
& =5.41 \times 10^{-12} \\
\lambda & =\frac{6.626 \times 10^{-34}}{5.41 \times 10^{-12}} \\
& =0.125 \mathrm{~nm}
\end{aligned}$
Q 7. No. of stereoisomers?
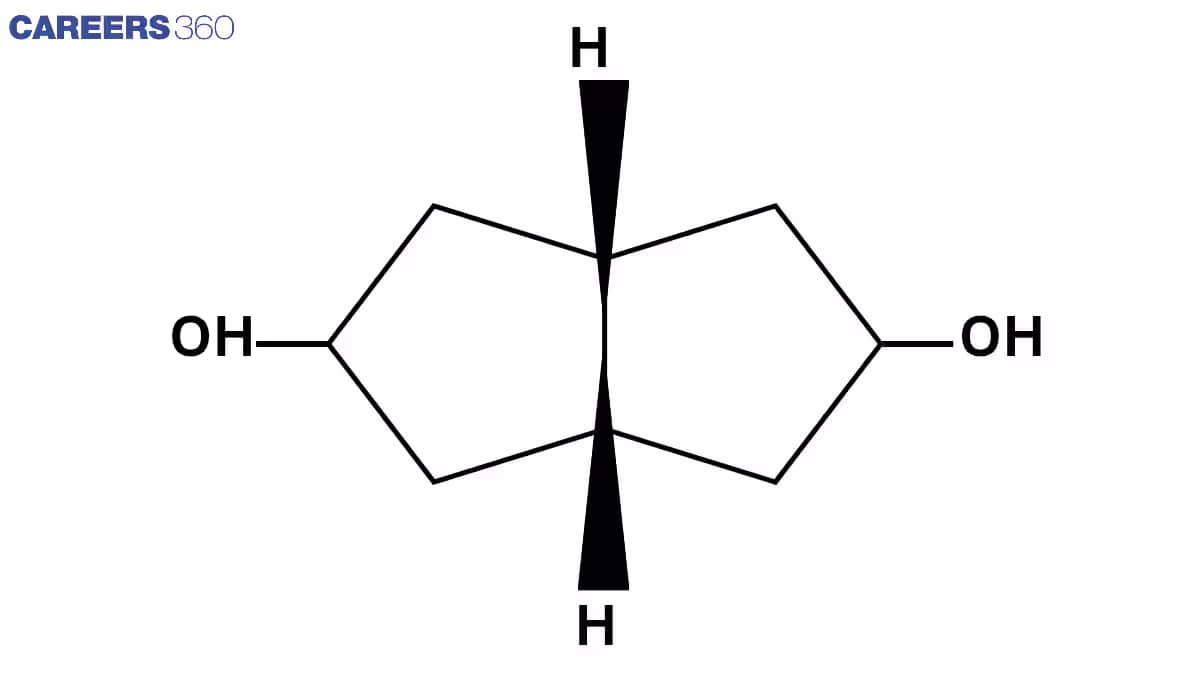
Ans. There are 3 optical centre
No. of Sterioisomer is $2^n=2^3=8$
According to the initial feedback of the candidates, the IIT JAM Chemistry 2025 question paper was of easy to moderate difficulty.
The inorganic and organic topics of the IIT JAM 2025 Chemistry question paper were of easy to moderate difficulty while the mathematic questions involved lengthy calculations and were seen on the difficult side. There were 6-7 such questions.
In the IIT JAM 2025 question paper, there were questions on topics such as Physical Chemistry, buffers, electrochemistry, and quantum concepts.
2 questions were asked about the concept of entropy in the IIT JAM Chemistry 2025 question paper.
There was an easy to moderate level difficulty question from the topic surface chemistry. Inorganic chemistry was on the easier side when compared to the other sections of the IIT JAM 2025 question paper.
In the 2025 IIT JAM question paper, there was one question asked on the topic of normalisation constant.
Analyzing the IIT JAM 2025 Chemistry question paper will help students identify important topics and focus their preparation accordingly.
Referring to the IIT JAM Chemistry question paper with answers PDF will help students understand the overall difficulty of the IIT 2025 JAM Chemistry question paper.
Subjects | Question Paper with Solution PDF |
Chemistry |
Section | Question Type | Total Questions | Marks per Question | Total Marks | Negative Marking |
A | Multiple Choice Questions (MCQs) | 30 | 10 questions: 1 mark each 20 questions: 2 marks each | 50 | 1 mark questions: -1/3 mark 2 mark questions: -2/3 mark |
B | Multiple Select Questions (MSQs) | 10 | 2 marks each | 20 | No negative marking No partial marking |
C | Numerical Answer Type (NAT) | 20 | 10 questions: 1 mark each 10 questions: 2 marks each | 30 | No negative marking |
Total | - | 60 | - | 100 | - |
Also Read,
Before heading towards writing the IIT JAM Chemistry examination 2025, it is always advisable to through the previous year’s IIT JAM Chemistry question paper analysis. This can give the candidates invaluable insights into the IIT JAM Chemistry examination and help them understand what they should expect from the IIT JAM 2025 question paper. To succeed in the IIT JAM Chemistry question paper 2025, candidates must strengthen their conceptual clarity and practice regularly using mock tests.
Overall Difficulty: The IIT JAM Chemistry 2024 paper was generally considered to be between moderate to difficult. While some sections were manageable, others tested deeper understanding and problem-solving skills, making the exam a bit more challenging than previous years.
Length of the Paper: Compared to the 2023 paper, the 2024 IIT JAM Chemistry exam was lengthier. This trend suggests that the 2025 exam might also be a lengthier paper with more questions, requiring better time management.
Weightage to Chemical Thermodynamics: The topic of Chemical Thermodynamics carried the highest weightage in the 2024 exam. This suggests that candidates are likely to remain an important topic in the IITJAM 2025 Chemistry question paper.
Good Attempt Criteria (25-30 Questions): Attempting around 25-30 questions with an accuracy of 95% was considered a strong attempt in the IITJAM Chemistry question paper 2024. This indicates that a focused approach, where candidates prioritize accuracy, is more beneficial than attempting many questions with lower precision.
Achieving 99 Percentile (30+ Questions): According to experts, attempting 30+ questions with a reasonable level of accuracy (a score of 60+) would likely guarantee a 99 percentile. This suggests that while accuracy is crucial, attempting a sufficient number of questions is also key to securing a top rank.
Given below is a set of questions from the IIT JAM Chemistry Question Paper 2024. This can help the candidates understand the type of questions which they can expect in the IIT JAM Chemistry Question Paper 2025 and the possible exam difficulty level.
Q.1 The following dipeptide derivative is used as an artificial sweetener:
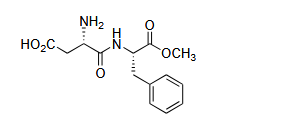
The constituent α-amino acids of this dipeptide are:
(A) Phenylalanine and glutamic acid.
(B) Phenylalanine and aspartic acid.
(C) Tyrosine and aspartic acid.
(D) Tyrosine and glutamic acid.
Q.2 The pair of proteins having heme core is:
(A) haemoglobin and myoglobin.
(B) hemerythrin and myoglobin.
(C) haemoglobin and hemocyanin.
(D) hemocyanin and hemerythrin.
Q.3 Identify the reaction for which, at equilibrium, a change in the volume of the closed reaction vessel at a constant temperature will not affect the extent of the reaction.
(A) CaCO₃(s) ⇌ CaO(s) + CO₂(g)
(B) H₂(g) + I₂(g) ⇌ 2HI(g
(C) 2NO₂(g) ⇌ N₂O₄(g)
(D) CO₂(s) ⇌ CO₂(g)
Q.4 [Co(NH₃)₅(SO₄)]Br and [Co(NH₃)₅Br]SO₄ are examples of:
(A) ionization isomers.
(B) linkage isomers.
(C) optical isomers.
(D) coordination isomers.
Q.1 The suitable synthetic route(s) for the following transformation
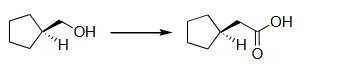
is/are:
(A) (i) para-toluenesulfonyl chloride (TsCl), pyridine; (ii) KI; (iii) Mg/Et₂O; (iv) CO₂; (v) aq. HCl
(B) (i) para-toluenesulfonyl chloride (TsCl), pyridine; (ii) KCN; (iii) conc. aq. NaOH, reflux; (iv) aq. HCl
(C) (i) CrO₃, H₂SO₄; (ii) SOCl₂; (iii) CH₂N₂; (iv) Ag₂O, H₂O
(D) (i) CrO₃, H₂SO₄; (ii) CH₂N₂
Q.2 The compound(s) which on reaction with CH₃MgBr followed by treatment with aqueous NH₄Cl would produce 1-methyl-1-phenyl ethanol as the major product is/are:
(A) methyl benzoate.
(B) phenyl acetate.
(C) acetaldehyde.
(D) acetophenone.
Q.3 The correct assumption(s) required to derive Langmuir adsorption isotherm is/are:
(A) Adsorption is limited to a monolayer on the adsorbing surface.
(B) All binding sites on the adsorbing surface are identical.
(C) Adsorption of a molecule on a site enhances the binding of other molecules on neighbouring sites.
(D) Rate of adsorption and rate of desorption are equal at equilibrium
Q.4 Consider the exothermic chemical reaction O₂(g) + 2H₂(g) ⇌ 2H₂O(g) at equilibrium in a closed container. The correct statement(s) is/are:
(A) At equilibrium, the introduction of a catalyst increases product formation.
(B) Equilibrium constant decreases with an increase in temperature.
(C) The equilibrium constant Kₚ increases with pressure.
(D) Decrease in the volume of the reaction vessel increases product formation.
Q.1 For the following compound
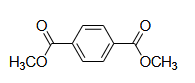
the number of signals expected in the ¹H NMR spectrum is _______.
Q.2 Exhaustive hydrogenation of the following compound
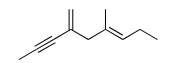
under Pd/C generates a saturated hydrocarbon as the product.
The number of stereoisomers possible for this product is ______.
Q.3 For a zero-order reaction P → Q, the concentration of P becomes half of its initial concentration in 30 minutes after starting the reaction.
The concentration of P becomes zero at ______ minutes.
(rounded off to the nearest integer)
Q.4 The magnitude of the energy difference between the energy levels n = 3 and n = 2 of a quantum particle of mass m in a box of length L is
Xh² / 8mL².
Then X = _______.
(rounded off to the nearest integer)
[Given: h is Planck’s constant and n denotes the quantum number.]
The Indian Institute of Technology, Delhi has created an IIT JAM Chemistry mock test for the IIT JAM Chemistry aspirants. It follows the same IIT JAM Chemistry exam syllabus and the difficulty levels. The candidates are strongly advised to attempt the official mock test to asses their preparation levels and make any changes to it if necessary.
Title | Download Link |
IIT JAM Chemistry Official Mock Test |
| Title | Download Link |
| IIT JAM 2025 Question Paper with Solutions | Download Now |
Note:
Frequently Asked Questions (FAQs)
Yes, NCERT is sufficient for building a solid foundation in IIT JAM Chemistry, as it covers basic concepts comprehensively. However, for advanced understanding, additional resources like "Physical Chemistry" by Peter Atkins and Julio de Paula, "Organic Chemistry" by Morrison and Boyd, and "Inorganic Chemistry" by Gary L. Miessler and Paul J. Fischer are recommended.
Yes. the IIT JAM exam can be given for two exams but the registration fee will increase for the additional paper.
Yes, it is possible to crack IIT JAM Chemistry in 3 months with dedicated preparation. Focus on understanding the core concepts, revising important topics, and practising numerical problems regularly. Solving previous years' papers and taking mock tests can also help improve speed and accuracy, ensuring better performance in the exam.
A good score in IIT JAM Chemistry paper is 70 or above, as it increases the chances of securing admission to top IITs or NITs.
IIT JAM Chemistry question paper is generally considered moderate to difficult, depending on a candidate's preparation and conceptual clarity. The exam tests in-depth knowledge of physical, organic, and inorganic chemistry, with questions often requiring strong analytical and problem-solving skills. Consistent study and practice are key to managing its challenges effectively.
On Question asked by student community
Hello Nisha. No IIT JAM and NEET are not the same level the reason is quite simple.
NEET is an exam for students after class 12th who want to become doctor. The questions are asked only from the class 11th and 12th syllabus of physics, chemistry and biology. The concepts
Hello,
Your exam result will still be generated after you take the IIT JAM exam .
But if you do not submit a valid EWS certificate , then:
Your EWS category benefit will be cancelled .
Your result may be treated as General category .
At the admission stage ,
Hello,
If your IIT JAM status shows “Under Scrutiny: Defect Rectification Done”, it means your correction is submitted and under review. Keep checking your portal, once verified, it’ll change to “Accepted” or “Found Defective.” Updates usually appear within 2–3 days before the portal closes.
Hope you understand.
For IIT JAM BIOTECHNOLOGY EXAMINATION the 4 subjects are PHYSICS, CHEMISTRY, MATHEMATICS AND BIOLOGY and the candidate must be graduated with other criteria. Nowhere in the eligibilities it is needed to have mathematics in +2 level.
You can check out the other eligibility in the brochure given below by Careers360.
Hello dear candidate ,
JAM exam is conducting in english only , no hindi option is available in this exam so there is no choice to giving the IIT JAM exam in Hindi , you must have to prepare in english for IIT JAM .
Hope this information is useful
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Master's programs in Sustainability Science and Practice; Climate Change Science and Practice; Urban Economic and Infrastructure Development; Human Development Policy and Practice
NAAC A+ Accredited | Among top 2% Universities Globally (QS World University Rankings 2026)
Highest CTC 30 LPA | #9 in Management Category by Times B-School | Merit-Based Scholarship Upto - 50 Crores
Last Date to Apply: 25th Feb | Ranked #45 Among Universities in India by NIRF | 1950+ Students Placed 91% Placement, 800+ Recruiters
MSc Finance and MSc International Management Admissions 2026 Now Open | Ranked Among the Top 100 Universities in the World by QS World University Rankings 2025 | Early Round 2 Applications Deadline: 29th Jan’26