Amity University-Noida BA Admissions 2026
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
CUET 2025 Chemistry Question Paper Analysis: The Chemistry exam for CUET 2025 was conducted between May 13 and June 3, 2025, as part of the Common University Entrance Test for undergraduate admissions to central and participating universities across India. This paper is crucial for candidates seeking admission to science-focused courses like B.Sc. Chemistry, B.Pharm, and related fields.
This Story also Contains

With the exam now been concluded, this article has been updated with a detailed CUET 2025 Chemistry question paper analysis, covering section-wise topic weightage, difficulty level, question types, and the overall emphasis on the Class 12 NCERT Chemistry syllabus. Aspirants can use this analysis to assess their performance and guide future preparation.
The Chemistry section of CUET is designed to test a candidate's conceptual clarity, analytical ability, and problem-solving skills. Covering all major areas—Physical, Organic, and Inorganic Chemistry—the exam includes questions that assess understanding of theory, formulas, and real-life applications based on the NCERT Class 12 curriculum.
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Last Date to Apply: 26th March | Ranked #45 Among Universities in India by NIRF | 1950+ Students Placed, 91% Placement, 800+ Recruiters
CUET 2025 Exam Pattern for Chemistry:
Aspects | Details |
Conducting Body | National Testing Agency (NTA) |
Sections | II – Domain Specific |
Type of Questions | Multiple Choice Questions (MCQs) |
Total Questions | 50 (for each subject including Chemistry) |
Duration | 60 minutes per subject |
Marking Scheme | +5 for each correct answer –1 for each incorrect answer 0 for unattempted |
Max. Number of Papers | Up to 5 test papers |
The CUET 2025 Chemistry question paper is designed to test students’ understanding of core concepts from NCERT and related syllabus. This detailed analysis of the CUET 2025 chemistry question paper highlights the difficulty level, important topics, and types of questions asked in multiple shifts, helping future aspirants prepare effectively for the exam.
Memory-based questions from CUET 2025 help students understand the type of questions that appeared in the actual exam. They are useful for identifying important chapters, repeated concepts, and question formats. Practicing these can boost confidence and improve exam readiness.
Below are the questions that came in CUET Chemistry exam 2025:
Q1.What is the result of the hydrolysis of cellulose?"
Q2.Which type of compound or mixture shows the Tyndall effect?
Q3. Write the nearest equation
Q4.Lactose is formed by the condensation of which two monosaccharides?"
Q5. Which of the following is NOT a type of isomerism?
Q6.e^x + e^y = e^(xy) so, dx/dy
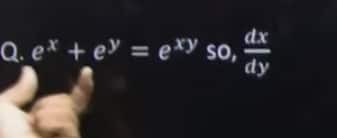
Q7.Structure of Tetraammine aqua chloride cobalt(III) chloride
Q8.Which of the following compounds will undergo the Hofmann reaction?
Q9 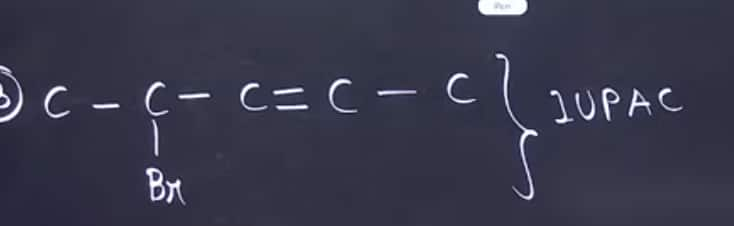
Q10.Write nearest equation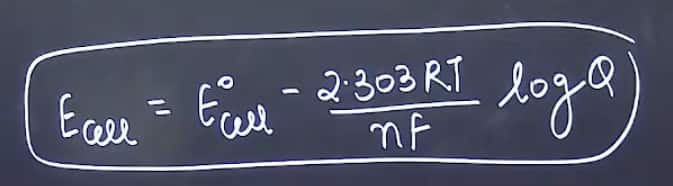
Paragraph question from chemical kinetics was asked
Question from IUPAC naming was asked
3–4 questions from coordination compounds were asked
Question from magnetic moment and Fe³⁺ complex was asked
Question from electrochemistry was asked
Question on Clemmensen reduction was asked
The paper was Easy to Moderate level with diverse chemistry topics covering molarity and PPM calculations that tested quantitative analytical skills in solution chemistry.
Organic chemistry featured matching questions on common naming systems, glucose and lactose hydrolysis reactions, testing carbohydrate chemistry knowledge.
Biochemistry section included questions on vitamin deficiency diseases and thyroxine hormone, connecting chemistry with biological applications.
Applied chemistry topics covered Gabriel phthalimide synthesis and picric acid properties, requiring knowledge of organic synthesis and aromatic compounds.
The paper balanced theoretical concepts with practical applications, emphasizing both calculation skills and factual recall across organic, inorganic, and biochemistry domains.
Students with strong foundation in solution chemistry, biomolecules, and organic reactions would have found the paper manageable for scoring.
The paper was Easy to Moderate level with physical chemistry featuring boiling point, molality, cell constant, and specific conductance questions testing solution and electrochemistry concepts.
Inorganic chemistry covered coordination numbers, oxidation numbers, F-block electronic configuration, and magnetic moment calculations, emphasizing transition metal chemistry.
Organic chemistry included naming reactions, organic reaction mechanisms, and amino acids matching questions, testing both reaction understanding and biomolecule knowledge.
The paper balanced theoretical concepts with numerical applications across all three chemistry branches, favoring students with comprehensive NCERT preparation.
Coordination chemistry and electrochemistry appeared prominently, making these topics crucial for achieving good scores in the examination.
Question was asked on IUPAC name of Metacresol
Question was asked on Formula of Freon
Question was asked from Swarts Reaction
Question was asked from Azo Coupling Reaction
Question was asked from SN2 Reaction in Aryl Halide
2 questions were asked from Boiling Point
2 questions were asked on Basic Nature
Question was asked on Application of Henry’s Law
Question was asked on Molality
Question was asked on Hybridisation
Question was asked from Biomolecules – Uracil Structure
Question was asked on Amino Acids – Match the Following
Easy to Moderate | Numerical, Conceptual, and Matching-Type Mix
A matching-type question from ideal and non-ideal solutions was included.
Colligative properties were tested through a formula-based numerical.
Chemical kinetics had a rate law question based on first-order reactions.
Molecularity was assessed through a statement-based conceptual question.
Electrochemistry had numerical-based application questions.
Coordination compounds included an IUPAC nomenclature question.
A question related to the structure and function of Cisplatin was asked.
Increasing order of unpaired electrons was tested conceptually.
Lanthanide contraction was directly asked.
A paragraph-based question focused on vitamins.
Organic chemistry included questions from phenol and benzoic acid compounds.
HVZ reaction and Tollens’ reagent-based questions were also present.
Difficulty level of the exam is Easy to Moderate
Direct Questions from NCERT resources
Match the following questions from Azeotrope and Ideal, and Non-Ideal solutions.
Statement-based questions from Complex Reactions.
Catalyst-related questions were frequently asked.
Graphical questions from Zero Order Reaction tested the kinetics understanding.
Electrochemistry
Paragraph and kinetics questions required conceptual clarity.
Faraday's Law numerical questions demanded quantitative problem-solving skills.
Match the following from Battery Cells covered electrochemical applications.
Inorganic Chemistry
Question from Inner and Outer Orbital theory.
Questions related to the Magnetic Moment test coordination compound properties.
Arrangement of oxidation rates required an understanding of redox reaction sequences.
Organic Chemistry
6-7 questions from Biomolecules, emphasizing structural knowledge.
Vitamin K deficiency diseases are tested for biochemical applications.
Glucose open chain structure questions examined carbohydrate stereochemistry.
Paper was easy to moderate
T50 given and T100 was asked
Formula of alum was asked
Question from double salt came
Rate of reaction related question came
SN2 reaction rate was asked
Aniline basic strength was asked
Coupling reaction question came
Named reactions matching question came
Anomers-related question was asked
The paper level was easy to moderate.
Around 35 questions were easy, and 8–10 were moderately difficult.
A question was asked from organic chemistry on the acidic nature of benzoic acid.
A question was asked about solutions, where urea’s density was given.
A question on the IUPAC naming of compounds was asked.
A question on the basic strength of a compound in aqueous medium was asked.
A matching-type question related to vitamin sources was asked.
A question about essential amino acids was asked.
A question on the structure of acidic amino acids was asked.
A question asking about the SN1 reactivity order was asked.
A numerical question was asked about colligative properties, based on depression in freezing point.
A theoretical question related to osmotic pressure was asked.
A question was asked regarding the chemical equation of a lead storage battery.
The Chemistry paper was of moderate difficulty, with questions spread evenly across different topics.
Numerical questions were present but were generally easy to moderate in difficulty.
The paper included questions from Physical Chemistry, especially colligative properties and chemical kinetics.
Questions on organic chemistry were mostly about IUPAC naming, reactions, and identifying functional groups.
Inorganic chemistry questions covered topics like vitamin deficiency diseases, periodic table properties, and some theory-based questions.
There were some questions requiring the use of formulas and conceptual understanding, not just rote memorization.
The paper had a mix of theoretical and application-based questions.
Electrochemistry questions appeared but were generally straightforward and formula-based.
Some questions involved conceptual reasoning rather than direct fact recall, testing deeper understanding.
Overall, students found the paper manageable if they had prepared well, especially on important topics like kinetics and organic reactions.
Students were advised to review important chapters such as colligative properties, kinetics, vitamin diseases, and IUPAC nomenclature.
The paper was balanced and did not have unexpected or extremely difficult questions.
The paper was easy to moderate in difficulty.
Questions were directly based on NCERT, with minimal deviation.
A comprehension-type paragraph was included from Electrochemistry and Kinetics.
Biomolecules had fewer questions than expected.
A Match the Following test measures knowledge of naming reactions.
There were 2 IUPAC naming reactions, checking basic nomenclature understanding.
A numerical/conceptual question on the conductance unit was asked.
One difficult question from Coordination Chemistry was included.
Considered the easiest among the PCM subjects in this shift.
The majority of questions were directly from NCERT, making it very familiar for prepared students.
Around 8-10 numerical questions were reported, but they were formula-based and straightforward.
Important topics included Solutions, Chemical Kinetics, Biomolecules, and Coordination Compounds.
Balanced mix of Organic, Inorganic, and Physical Chemistry.
Students found the paper comfortable and predictable.
Overall Difficulty Level: Easy to Moderate
Topic Weightage: Balanced and similar to other shifts
Sandmeyer reaction question tested the identification of products.
Hinsberg reagent question asked about its name and use in separation.
Common naming of compounds, especially those containing the –CH₂COOH group.
Maltose hydrolysis question involved basic product knowledge.
Sucrose is identified as a non-reducing sugar.
DNA and RNA questions focused on the identification of nitrogenous bases.
A CFSE-based question appeared from Coordination Compounds.
d- and f-block elements had around 4 questions, mainly on basic physical properties.
Color identification of transition metal compounds was tested.
A few questions were on periodic trends and general inorganic facts.
Molality calculation is tested under the Solutions chapter.
The solubility concept was tested with conceptual MCQs.
Isotonic, hypertonic, and hypotonic solutions appeared in a matching-type question.
Chemical Kinetics had statement-based questions on zero and first-order reactions.
One question on the effect and nature of catalysts.
A paragraph-based question tested understanding of Electrochemistry.
Nernst equation application was assessed in a theoretical context.
Question on fuel cells tested practical application knowledge.
Slightly concept-heavy and required deeper understanding.
Many questions were not direct and demanded the application of learned concepts.
Very few numerical-type MCQs were present.
The paper was mostly theoretical with minimal calculation-based problems.
The Chemistry paper closely mirrored the NCERT syllabus, making it approachable for well-prepared students.
Organic Chemistry dominated the paper, with Inorganic following; Physical Chemistry had fewer questions.
Most questions were factual, directly testing core concepts and reactions from the NCERT.
The paper didn’t introduce unexpected twists and stuck to standard patterns.
Students who had revised the NCERT thoroughly were able to handle the section with ease.
On the whole, the Chemistry section was viewed as easy to moderate in difficulty.
The chemistry paper was considered easy to moderate in difficulty.
Most questions were factual and directly from the NCERT textbook.
Equal weightage was given to Inorganic and Organic Chemistry.
Important topics included Coordination Compounds and Aldehydes/Ketones.
Physical Chemistry questions were basic and involved simple formula-based problems.
The paper was seen as straightforward and scored by students.
Those with a strong memory of the NCERT theory found the paper easy to handle.
Overall Level: Easy to Moderate
Conceptual and theory-based questions dominated the paper.
No out-of-syllabus or unseen questions were asked.
Options were confusing, which made certain questions tricky.
Many questions were similar to previous year questions (PYQs).
Questions appeared from the chapter on Solutions.
Specific questions from Freezing Point and Raoult’s Law were included.
A question from the Lucas Test was present.
Isomerism and optical activity/inactivity were tested.
A structural question on Sucrose was asked.
Overall, the paper was theory-heavy with few numerical problems.
The Chemistry paper was moderate in difficulty.
It included a mix of conceptual and application-based questions.
Most questions were NCERT-based, though a few required deeper understanding.
Around 2–3 questions came from Organic Chemistry, covering reaction mechanisms and IUPAC naming.
Physical Chemistry questions involved numerical problems from the Mole Concept, Thermodynamics, and Equilibrium.
Inorganic Chemistry had 2 questions focusing on Periodic Table trends and Coordination Compounds.
Paper difficulty ranged from easy to moderate.
Organic chemistry formed the bulk with approximately 25-27 questions.
Inorganic chemistry questions numbered around 10-11.
Physical chemistry included 12-13 questions.
Important questions/topics:
Hoffman Bromamide reaction and ether reaction with HI.
Hydrolysis of cellulose, highlighting organic reaction mechanisms.
Magnetic moment-related questions assessing electron configurations.
Isotonic solution concept from NCERT Exemplar.
Animal starch-related question to test biochemical knowledge.
Electron configuration-based question on unpaired electrons.
Question related to propene chemistry
The paper was moderate with a slight inclination towards Physical and Organic Chemistry.
Important topics included Chemical Kinetics, Electrochemistry, Coordination Compounds, and Biomolecules.
Numerical problems were straightforward but required accuracy.
Assertion-Reason and statement-based questions tested in-depth understanding.
Organic chemistry reactions and mechanism-based questions were prominent.
NCERT-based factual questions were present, focusing on direct knowledge.
Students found the section balanced but slightly lengthy.
The paper was easy to moderate overall.
Organic Chemistry
Highest weightage with 27 questions.
IUPAC naming questions included.
Werner’s theory question was asked.
Color-related question repeated from 13th May.
IUPAC rule sequence-based question appeared.
Matching question on Tollen’s, Fehling’s, and Schiff’s reagents.
Nucleophilic substitution reaction included.
A cumene process-based question was asked.
Coordination Compounds
One full paragraph-type question with 7–8 sub-questions based on it.
Physical Chemistry
8 to 12 questions are asked.
Van’t Hoff factor and its applications included.
Solubility question related to Henry’s Law.
Electrochemistry questions based on the types of cells.
Chemical Kinetics questions included.
Pseudo first-order reaction-related question was asked.
Acidic strength comparison question.
Carbonic acid formula asked.
Inorganic Chemistry (D & F Block)
12 questions asked.
Glucose-related question repeated (3rd time overall).
Question on 3d series: second-highest ionization energy.
A question was asked based on Van’t Hoff factor, focusing on its relation to colligative properties.
Rate of reaction and order of reaction-based numerical questions were asked from chemical kinetics.
Conceptual questions on units appeared, including match-the-following format questions.
Multi-statement-based tricky questions tested a deeper understanding of various chemical principles.
A passage-based question on electrolytic cells was included, assessing comprehension of the topic.
A question related to the chelate effect was asked in coordination chemistry, focusing on complex stability.
IUPAC nomenclature questions required identifying the correct names from the given structures.
A question on ambidentate ligands was asked, testing knowledge of ligands bonding through different atoms.
Magnetic moment calculation was tested based on unpaired electrons in transition metal ions.
Around 2-3 questions were from the d and f block elements, covering their properties and reactions.
A reaction-based question was asked on the Rosenmund reduction reaction.
Conceptual questions were asked about Tollens’ test, Fehling’s test, and HVZ reaction.
SN1 reactivity order was tested by comparing carbocation stability in different substrates.
A set of reactions was provided, asking to identify which involves nitration.
A straightforward question on sucrose hydrolysis and its resulting products was included.
A question was asked based on the structure of glucopyranose, focusing on carbohydrate chemistry.
A conceptual question tested knowledge of boiling point order among ethane derivatives based on intermolecular forces.
The CUET 2025 Chemistry paper was rated easy to moderate in difficulty, making it accessible for students thorough with NCERT concepts.
The nature of questions was direct and NCERT-based, with minimal tricky or application-heavy problems.
Students reported the paper as scoring for those who revised the NCERT Chemistry Class 12 chapters properly.
Identification of the compound exhibiting the Tyndall effect.
A question on the order of basicity among amines, testing fundamental organic chemistry concepts.
An electrolysis-based conceptual question, assessing knowledge of electrochemical processes.
A numerical problem on molarity, involving direct formula application.
Mechanism-based question on SN1 vs SN2 reaction pathways, requiring a clear understanding of reaction intermediates.
The overall difficulty level of the Chemistry paper was easy to moderate, making it scoring for well-prepared students.
A major focus was on organic chemistry, with 24 questions, testing concepts like mechanisms, reactions, and conversions.
Coordination compounds were significant, with 7 questions, including topics like IUPAC naming and properties.
Biomolecules and solutions had limited weightage, with 2 questions each, focusing on basic concepts like molality and Van’t Hoff factor.
Chemical kinetics appeared in 3 questions, with emphasis on rate constant units and reaction rates.
Electrochemistry had 4 questions, including numerical concepts like EMF comparison and Nernst equation applications.
The D- and F-block elements were tested through 4 questions, focusing on their properties and applications.
A question involved calculating molality from % weight by weight, testing the practical application of solution concentration formulas.
Despite the variety of topics, the paper had fewer numerical questions, reducing the calculative load for students.
Organic Chemistry had reaction mechanisms, IUPAC nomenclature, and isomerism; moderate level.
Inorganic Chemistry focused on periodic table trends, coordination compounds, and metallurgy.
Physical Chemistry questions on the mole concept, thermodynamics, and equilibrium were calculative.
Surface Chemistry and Biomolecules topics were covered with straightforward questions.
Chemical Bonding and Molecular Structure had conceptual questions.
Practical-based questions on salt analysis and titration were included.
Students found Chemistry balanced with equal weightage to all units
Many students tend to skip paper analysis, but it’s an essential tool for identifying patterns, predicting trends, and fine-tuning preparation. Here’s how it helps:
Highlights High-Weightage Units: Reveals whether topics like Chemical Bonding, Coordination Compounds, or Equilibrium had more emphasis
Numericals vs. Theory: Understand the ratio of formula-based questions to direct theoretical ones
Repetition of Concepts: Pinpoint frequently asked ideas such as Mole Concept, Redox Reactions, or p-Block Elements
Application-Oriented Thinking: Shows whether questions required mere recall or real understanding and application
Class 11 vs. 12 Weightage: Helps balance study plans based on year-wise distribution
Time Management Insight: Know which sections consumed the most time or required quicker calculation.
Frequently Asked Questions (FAQs)
Yes, NCERT is the primary source. For enhanced practice, refer to PYQs, mock tests, and sample papers for speed and accuracy.
The difficulty level is usually easy to moderate. While some numerical problems may be tricky, most questions are manageable with a strong foundation in NCERT.
The section features MCQs that are either theory-based, numerically driven, or conceptually applied. Students may be asked to recall facts, perform calculations, or apply scientific principles.
The syllabus includes Class 12 NCERT Chemistry topics like Chemical Bonding, Equilibrium, Thermodynamics, Redox Reactions, Coordination Compounds, Polymers, Biomolecules, and Environmental Chemistry.
On Question asked by student community
To prepare for the CUET UG exam, it is important to understand that while it is based on the Class 12 NCERT curriculum, the CUET syllabus is structured very differently from the CBSE Board exams
NTA has released the CUET UG 2026 application form on January 3 at cuet.nta.nic.in. Aspirants can fill and submit the CUET UG form 2026 till January 31. The candidates must provide their academic, personal, and contact information in the CUET UG 2026 application form. Prior to submission, it is important
A mismatch in father's name on official documents (like 10th marksheet vs. Aadhaar) will not lead to an immediate rejection of CUET application form, but it can cause significant problems during the document verification stage at universities. The National Testing Agency provides a CUET UG correction window to fix such
Hello
The application process for the Common University Entrance Test (CUET) UG 2026 has officially begun. The National Testing Agency (NTA) released the application form on January 3, 2026. If you want to join top central universities like Delhi University (DU), BHU, or JNU, you must apply before the deadline.
Hello
Yes, it is fine to choose Political Science and History as your domain subjects, English as your language, and GAT in CUET for BA Ancient Indian Culture & Archaeology at BHU. These subjects are fully accepted and will not affect your eligibility for the course.
Among top 100 Universities Globally in the Times Higher Education (THE) Interdisciplinary Science Rankings 2026
Bristol's expertise meets Mumbai's innovation. Admissions open for UG & PG programmes
UG & PG Admissions open for CS/AI/Business/Economics & other programmes.
Recognized as Category-1 Deemed to be University by UGC | 41,000 + Alumni Imprints Globally | Students from over 20+ countries
Study at a world-renowned UK university in India | Admissions open for UG & PG programs.
100% Placements Assistance | 1200+ Recruiters